Monitoring alkalinity and hardness
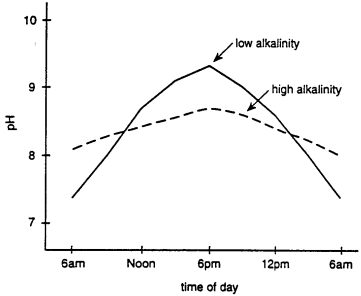
Alkalinity refers to the total amount of bases in water expressed in mg/l of equivalent calcium carbonate. A base is a substance that releases hydroxyl ions (OH-) when dissolved in water. In most waters these bases are principally bicarbonate (HCO3) ions and carbonate ions (CO32-). These ions are the buffers in water; that is they buffer the water against sudden changes in pH. They can do this by absorbing hydrogen ions when the water is acid and releasing them when the water becomes basic. Waters of low alkalinity (<20 mg/l) are poorly buffered, and the removal of carbon dioxide (CO2) during photosynthesis results in rapidly rising pH. Waters, with greater than 20 mg/l alkalinity have greater buffering capacity and prevent large fluctuations in pH during photosynthesis (Fig. 1).
Fig. 1. The effect of buffering on pH at low and high alkalinities
Hardness is the concentration of metal ions (primarily calcium and magnesium) expressed in mg/l of equivalent calcium carbonate. Alkalinity and hardness values are normally similar to magnitude because calcium, magnesium, bicarbonate, and carbonate ions in water are derived in equivalent quantities from the solution of limestone in geological deposits. However, in some waters alkalinity may exceed its hardness and vice versa. If alkalinity is high and hardness low, pH may rise to very high levels (greater than 10.5) during periods of rapid photosynthesis.
Waters are often categorised according to degrees of hardness as follows:
- 0-75 mg/l - soft
- 75-150 mg/l - moderately hard
- 150-300 mg/l - hard
- over 300 mg/l - very hard
Alkalinity and hardness are not greatly affected by biological activity or aquacultural operations, and the initial concentrations in ponds are determined by their level in the water supply; any changes are largely the result of rainfall and evaporation. Desirable levels for fish culture generally fall within the range of 20-300 mg/l. If total alkalinity and total hardness are too low, they may be raised by liming. However, there is no practical way of decreasing alkalinity and hardness when they are above desirable levels.
As a general rule, the most productive waters for fish culture have a hardness and alkalinity of approximately the same magnitude. For example, a water with an alkalinity of 100 mg/l and hardness of 10 mg/l is not as good for fish culture as water in which the alkalinity is 100 mg/l and the hardness is 100 mg/l. Greater production does not result directly from higher levels of hardness and alkalinity per se, but from the higher concentrations of phosphorus and other essential elements that increase along with hardness and alkalinity.