Monitoring pH
The pH is the measure of the hydrogen ion concentration in soil or water. An ion is an electrically charged atom. Water exists as a balance between hydrogen ions (H+) and hydroxyl ions (OH-) and has the formula H20. The pH scale ranges from 0-14 with 7 neutral. When there are more hydrogen ions (H+) present the pH will be lower than 7 and the water acidic. The water is basic (alkaline) when there are more hydroxyl ions (OH-).
Silver perch grow best in a pH range of 6.5 to 9. Fig. 1 shows the relationships of pH of pond waters to their suitability for fish.
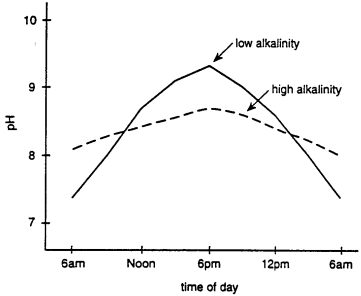
Carbon dioxide has an acidic reaction in water. The pH in ponds rises during the day because phytoplankton and other aquatic plants remove carbon dioxide from the water during the process of photosynthesis. The pH decreases at night because of respiration and production of carbon dioxide by all organisms.
Signs of Sub-optimal pH:
- increase of mucus on gill surfaces
- damage to eye lens and cornea
- abnormal swimming behaviour
- fin fray
- death
- poor phytoplankton and zooplankton growth.
Effects of Sub-Optimal pH:
- stress
- increased susceptibility to disease
- low production levels
- poor growth.
Causes of Sub-Optimal pH:
- acidic water and soils
- acid sulphate soils
- poorly buffered water, i.e. low alkalinity (<20 mg/l)
- waters having high alkalinity and low hardness
- acid rain.
Sub-optimal pH - What can I do?
To decrease high pH:
- flush the pond
- reduce feeding rates to lower nutrient input and plant growth
- ponds built in acid sulphate soils should be
- refilled immediately to prevent drying
- built no deeper than necessary
- grassed on the walls
- limed on the walls
- add gypsum (CaSO4) to increase the calcium concentration
- add alum (AlSO4) for immediate reduction of pH to avert imminent fish mortality.
To increase pH:
- lime the ponds
- increase phytoplankton abundance by fertilising