Cation exchange capacity
Cation exchange capacity (CEC) is a useful indicator of soil fertility because it shows the soil's ability to supply three important plant nutrients: calcium, magnesium and potassium.
Cations
What CEC actually measures is the soil's ability to hold cations by electrical attraction. Cations are positively charged elements, the positive charge indicated by a + sign after the element symbol. The number of + signs indicates the amount of charge the element possesses.
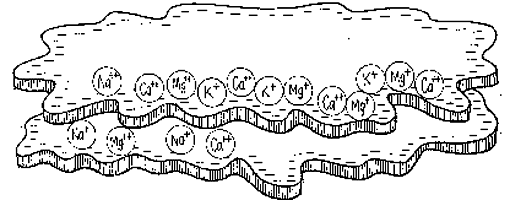
The five most abundant exchangeable cations in the soil are calcium (Ca++ ), magnesium (Mg++), potassium (K+), sodium (Na+) and aluminium (Al+++).
Cations are held by negatively charged particles of clay and humus called colloids. Colloids consist of thin, flat plates, and for their size have a comparatively large surface area. For this reason they are capable of holding enormous quantities of cations. They act as a storehouse of nutrients for plant roots.
As plant roots take up cations, other cations in the soil water replace them on the colloid. If there is a concentration of one particular cation in the soil water, those cations will force other cations off the colloid and take their place.
The stronger the colloid's negative charge, the greater its capacity to hold and exchange cations, hence the term cation exchange capacity (CEC).
CEC measurement
Concentrations of cations are expressed in centimoles of positive charge per kilogram of soil (cmol(+)/kg). This measurement is equivalent to the previously used unit me/100 g.
Adding the concentrations of each cation gives you an estimate of the CEC figure. A figure above 10 cmol(+)/kg is preferred for plant production. Soils with high levels of swelling clay and organic matter can have a CEC of 30 cmol(+)/kg or more.
The five exchangeable cations are also shown in soil test results as percentages of CEC. The desirable ranges for them are: calcium 65–80% of CEC, magnesium 10–15%, potassium 1–5%, sodium 0–1% and aluminium 0%.
pH and CEC
Soil pH is important for CEC because as pH increases (becomes less acid), the number of negative charges on the colloids increase, thereby increasing CEC.
CEC levels
Humus
CEC varies according to the type of soil. Humus, the end product of decomposed organic matter, has the highest CEC value because organic matter colloids have large quantities of negative charges. Humus has a CEC two to five times greater than montmorillonite clay and up to 30 times greater than kaolinite clay, so is very important in improving soil fertility.
Clay
Clay has a great capacity to attract and hold cations because of its chemical structure. However, CEC varies according to the type of clay. It is highest in montmorillonite clay, found in chocolate soils and black puggy alluvials. It is lowest in heavily weathered kaolinite clay, found in krasnozem soils, and slightly higher in the less weathered illite clay. Low CEC values can be improved by adding organic matter.
Sand
Sand has no capacity to exchange cations because it has no electrical charge. This means sandy soils such as podzolic topsoils have very low CEC, but this can be improved by adding organic matter.
Aluminium and sodium
Aluminium (Al+++) and sodium (Na++) cations are not plant nutrients, so are not wanted by the plant. Aluminium is not present as a cation when soil pH (CaCl2) is over 5 because it is precipitated out of the soil solution. It is only at pH (CaCl2) levels below 5 that it may become available as a cation, and under 4.5 may become available in toxic levels, displacing other cations from the clay or humus colloids. This is one reason why it is important to maintain pH levels at 5.0 or more.
When exchangeable sodium is present in quantities greater than about 5% of the CEC, it makes the clay particles unstable in rainwater. This shows up as dispersion or cloudiness in water. Dispersive soils have poor water entry and drainage and set hard on drying.
Average CEC of North Coast soils
| Soil | CEC (cmol(+)/kg) |
|---|---|
| Krasnozem | |
| High pH and high organic matter | 10–20 |
| Low pH and low organic matter | 2–6 |
| Chocolate | |
| High pH and high organic matter | 30-40 |
| Low pH and low organic matter | 3-7 |
| Podzolic | 3–10 |
| Alluvial | |
| Light and sandy | 10-20 |
| Heavy clay | 20-30 |
| Dune sand | 0-5 |
Leaching
If a soil has a low CEC and high sodium levels, up to half the cations in the soil may be in the water around the soil particles, and not actually held by the particles. These cations are very susceptible to being leached or drained away in the soil water.
Soils with a high CEC have a much lower percentage of cations in the soil water, so are far less susceptible to nutrient loss by leaching.
Improving CEC
You can improve CEC in weathered soils by adding lime and raising the pH. Otherwise, adding organic matter is the most effective way of improving the CEC of your soil. This can be done with permanent pasture, regular slashing, green manure crops, leaving crop stubbles to rot, rotating crops or pasture, and the addition of mulch and manure.
From the Soil Sense leaflet 3/93, Agdex 530 produced by Rebecca Lines-Kelly, formerly soils media officer, Wollongbar Agricultural Institute, for CaLM and NSW Agriculture, North Coast region, under the National Landcare Program, September 1993.