What is an acidic soil?
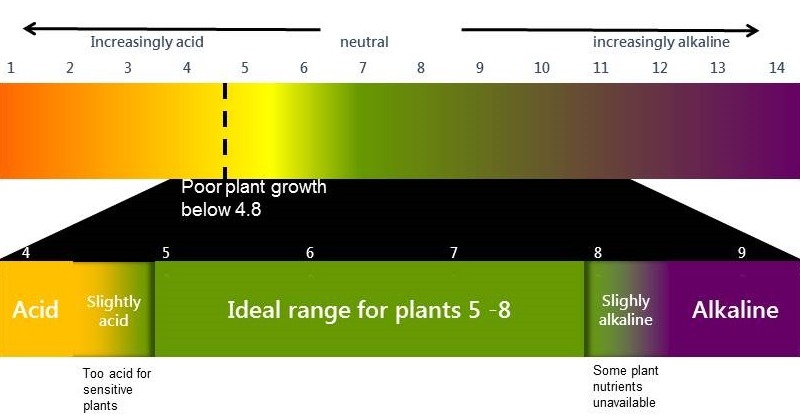
- Technically an acidic soil is one with a soil pH (CaCl2) below 4.8 in the root zone (0-20cm).
- A soil pH (CaCl2) between 5.5 and 8.0 provides the best conditions for most agricultural plants.
- Sometimes soil acidity is a natural characteristic of the soil which may be difficult to ameliorate economically.
How can I tell if soil acidity is a problem?
Although reduced production is often the end result, the visual signs of soil acidity are more subtle than the clearly visible symptoms of other soil degradation. A soil test is the most reliable way to assess if a soil is acidic.
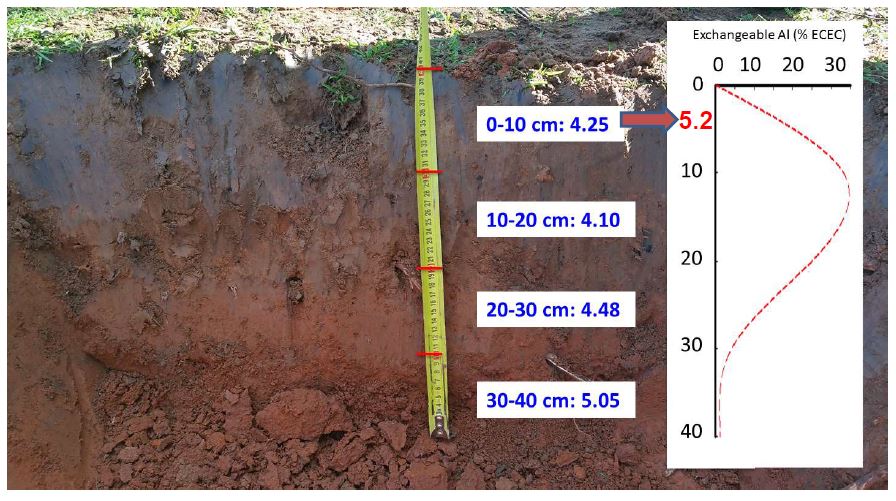
The only reliable way to determine if a paddock has an acidic subsurface soil is to sample and analyse the 0-5cm, 5-10cm, 10–15 cm, 15-20 cm, and 20–30 cm layers.
A comprehensive chemical analysis of the surface soil layers will give information to assist in determining if a crop or pasture will be affected by acidity. In order to assess whether acid sensitive crops will be affected by subsurface acidity, it is recommended that soil be tested for pH below 10cm, especially where reduced tillage has been used .
Read Signs and symptoms for other indicators
Testing
A field test using a field soil pH kit or meter is a good place to start. It is simple to carry out and will indicate if further investigation with a full laboratory test is required and at what intervals samples should be taken. This level of detail is required to decide which management strategies to employ and helps in species selection.
There are two accepted ways to measure soil pH: one in water and one in calcium chloride. The pH measured in calcium chloride is on average 0.5 to 0.8 units less than pH measured in water, although the difference can vary from nil to 2.0 for different soils, soil textures and salt content. In this publication pH is nominated as pH(CaCl2)
REMEMBER:
The pH scale is logarithmic, which means that every point is an order of magnitude different from that below or above it. For the pH scale in particular this means that a pH of 4 is 10 times more acid than 5 and 100 times more than a pH of 6.
Field colourmetric test uses the pH in water method
Plant growth and the pH (CaCl2) scale
If the pH(CaCl2) drops below 5.0, plants that are highly sensitive to acidity, such as some legumes and barley, are adversely affected. Plants that are more tolerant of acidity continue to grow normally until the pHCaCl2 falls below 4.8. However it is important to note that often Rhizobium bacteria are more sensitive to soil acidity than the host plant. This means nodulation and nitrogen fixation will be impaired before the host plant is affected (see). Not only does this have a negative effect on the legume itself but the lack of effective nodulation reduces the nitrogen contribution of legumes in both crop and pasture systems.
Below pHCaCl2 4.4 most plants, except the very highly acid tolerant plants like oats, narrow leaf lupins and the native pasture grass Microlaena spp, show a significant reduction in production. Even in tolerant species productive capacity will be affected by soil acidity. Even if soil acidity does not result in plant death, production will be suboptimal.
Soil acidity throughout the profile
Research has shown the process of soil acidification within the soil profile results in a stratification of soil pH. This means that the topmost surface of the soil profile (0-5cm) will often have a higher pH than those layers below. But it is these deeper layers that are important for plants to achieve their productive potential, as it is where most plant roots grow. When acidification is not managed it becomes more and more difficult to remediate. The greater the depth of the acidic layer, the greater the effect on plant growth and the more difficult it is to correct. Untreated subsurface soil acidity results long term degradation of the soil.
Whilst models showing the chemical process of soil acidification are very good at explaining the chemical causes, these models do not account for the three dimensional nature of soil in the paddock. They do not account for movement of acidity within the soil profile nor the fact that a soil profile is not uniform. This spatial variation results in stratification of soil pH down the soil profile in increments as small as 0-5cm, 5-10cm, 10-15cm etc which can be exacerbated by modern farming techniques such as reduced tillage. For further information on causes of soil acidity and stratification see Causes and video below.