Is Animal Ethics Committee approval required for your project?
If your project involves animals and it meets the definition of “Animal Research” for “recognised research purposes” as defined by the Animal Research Act 1985, then Animal Ethics approval is likely to be required.
What is animal research and recognised research purposes?
Animal Research
The Act defines animal research as any procedure, test, experiment, inquiry, investigation or study in connection with which an animal is used and, without limiting the generality of the foregoing, includes any procedure, test, experiment, inquiry, investigation or study in the course of which:
(a) an animal is subjected to:
(i) surgical, medical, psychological, biological, chemical or physical treatment,
(ii) abnormal conditions of heat, cold, light, dark, confinement, noise, isolation or overcrowding,
(iii) abnormal dietary conditions, or
(iv) electric shock or radiation treatment, or
(b) any material or substance is extracted or derived from the body of an animal.
Animal research does not include any procedure, test, experiment, inquiry, investigation or study which is carried out in the course of:
(c) the administration of veterinary treatment to an animal for the purpose of protecting the welfare of the animal, or
(d) the conduct of normal animal husbandry operations.
Recognised Research Purposes
The object of the Act is to protect the welfare of animals used in connection with research and authorisations provided under the Act are granted only for recognised research purposes. Recognised research purposes include purposes involving the use of animals for research, teaching, testing and the production of biological products.
Under the Act, a recognised research purpose means:
(a) the purpose of acquiring, demonstrating or developing knowledge in the field of medical, veterinary, agricultural, behavioural or biological science,
(b) the purpose of acquiring, demonstrating, exercising or developing techniques used in the practice of medical, veterinary, agricultural, behavioural or biological science,
(c) the purpose of developing or testing substances intended for therapeutic use (within the meaning of the Therapeutic Goods Act 1989 of the Commonwealth), or
(d) any purpose prescribed for the purposes of this paragraph.
Do you need to be accredited as an Animal Research Establishment or a Licenced Animal Supplier?
Under the Act, corporations which use animals in research and teaching in NSW must apply to DPIRD Compliance Systems & Accreditation Programs for Accreditation as an Animal Research Establishment. This includes government organisations such as local councils and government departments conducting research within NSW. Corporations are defined as businesses registered with ASIC and have an Australian Company Number (ACN). Please note that wildlife surveys are considered as animal research so if your business is conducting wildlife surveys and has an ACN, the business is required to be accredited under the Act.
Similarly, a person who supplies animals for use in research and teaching in NSW must apply to DPIRD Compliance Systems & Accreditation Programs for an Animal Supplier’s Licence. Please note that holders of Animal Supply Licences also need to apply to an Animal Ethics Committee (AEC) for approval to supervise their supply of animals for research purposes.
For further information on accreditation, please see the Animal Ethics Infolink or email bfs.admin@dpi.nsw.gov.au
The Secretary’s ACEC will only oversee projects for individuals or businesses that do not require accreditation under Division 2 of the Act or for small corporations which are accredited under the Act as an Animal Research Establishment and or are a Licenced Animal Supplier but do not have their own AEC. Please see the FAQs relating to the Secretary's ACEC Use Criteria for further details.
Animal research project approval process explained
The use of animals in research and teaching, such as field trials, product testing, the production of biological products, wildlife surveys, environmental studies, and observational studies of wildlife may only commence when an Animal Ethics Committee (AEC) has reviewed and approved an application to conduct research; the approval from the AEC has been received in writing and an Animal Research Authority (ARA) has been issued and received. Animal research in NSW can only be conducted under a current ARA.
Under the NSW Animal Research legislation:
- in order to conduct animal research in NSW, researchers must submit an application to an AEC for project approval prior to commencing research.
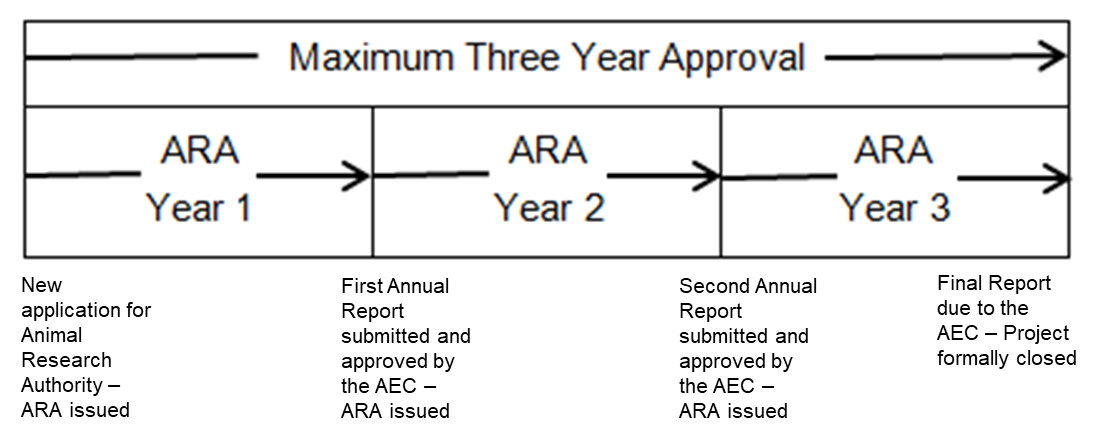
- the maximum time period for project approval is usually up to three years.
- an Animal Research Authority (ARA) is issued for a project once it has been approved by the AEC. Following receipt of the ARA by the researcher, the project may commence.
- the ARA lasts for maximum 12-month period under the NSW Animal Research legislation.
- an ARA can be renewed by providing an Annual Report for the project for AEC approval.
- the Annual Report must be submitted in time for the AEC to be able to review and approve it at a meeting scheduled prior to the project ARA expiry date. This allows the AEC to review the report and approve the project to continue for another 12 months and issue a new Authority before the expiry date.
- if you wish to continue a project after the 3-year period, you must submit a new application for the project.
- at the end of the project it is mandatory to submit a Final Report.
Difference between Animal Research Project Approval Period and Animal Research Authority approval period.
Animal Research Project Approval Period
In order to conduct animal research in NSW, including fauna surveys to determine presence and prevalence of fauna at locations, researchers must submit an application to an Animal Ethics Committee (AEC) for project approval prior to commencing research. If the AEC approves the project, it approves it for a certain period of time – usually 3 years.
Once the project has been approved by the AEC, an Animal Research Authority (ARA) is issued for a project for 12 months. Following receipt of the ARA by the researcher, the project may commence.
During this project approval period, the researcher is required to submit annual reports prior to each anniversary of the project approval date.
At the end of this project approval period, if the researcher wishes to continue to the project, they need to submit a new project application form to the AEC for approval. Please note, any project approvals, including modifications, do not roll over into the next project approval period. The new application is a complete restart and reset of the project.
If the researcher does not want to continue the project at or before the end of the project approval period a final report must be submitted to the AEC.
Animal Research Authority Approval Period
The Animal Research Authority (ARA) is the legal document which gives authority for animal research to occur under the NSW Animal Research Act 1985. It certifies an Animal Ethics Committee has approved the project and the procedures described in the project application. Only those people named on an ARA can undertake the procedures approved in the project (with the exception of C Class Bird and Bat Banders). Only procedures that have been approved in the project application can be carried out under this Authority,
Under the animal research legislation ARAs remain in force for a maximum 12 months and are only reissued for another 12 months following AEC approval of an annual report. Annual reports provide details of activities undertaken during the Animal Research Authority approval period.
The Annual Report must be submitted in time for the AEC to be able to review and approve it at a meeting scheduled prior to the project ARA expiry date. This allows the AEC to review the report and approve the project to continue for another 12 months and issue a new Authority before the expiry date. As dates of meetings and dates of ARA expiry do not usually align perfectly, this means annual reports often need to be submitted before the full 12 months has expired.
Late submission of annual reports will result in gaps in approval dates as Animal Research Authorities are not back dated.
If an annual report is not submitted within 3 months of the due date, it is assumed the researcher does not want to continue with the project and the project will be closed.
In summary – Projects are approved for 3 years, however, ARAs only last for 12 months. New annual ARAs are only reissued after the AEC has received, reviewed and approved the Annual Report for the project.
Note: It is an offence under the Act to conduct animal research or teaching without the possession of a current ARA.
See Figure 1 for details.
Figure 1. Approval Periods
Process for new researchers applying to the Secretary’s Animal Care and Ethics Committee for project approval
The process of applying to conduct animal research in NSW is different depending on whether the research will be done by:
- An individual or a business that is not a corporation (i.e., does not have an ACN registered with ASIC). Accreditation under the Act is not required prior to conducting animals research, however, application to an Animal Ethics Committee for approval of the animal research project (followed by being issued an Animal Research Authority) is required.
- A business that is a corporation (i.e., does have an ACN registered with ASIC). Accreditation under the Act is required prior to conducting animal research. Application to an Animal Ethics Committee for approval of the animal research project (followed by being issued an Animal Research Authority) is also required.
The processes of application for accreditation under the Act and application for an Animal Research Authority are two completely separate processes.
If you would like to use the Secretary’s Animal Care and Ethics Committee (ACEC) to apply for an Animal Research Authority, the process for new applicants is described below and also in Guidance for new researchers wanting to use the Secretary’s ACEC.
Please also refer to the:
FAQS re Secretary's ACEC Use Criteria
FAQs re Secretary's ACEC new fee schedule
Steps that must be undertaken include:
- Contact the DPIRD Compliance Systems and Accreditation Program unit to see if you or your business needs to be accredited under the Animal Research Act 1985 to be able to lawfully conduct animal research within NSW. If you have an ACN, this will likely be the case.
Please email bfs.admin@dpi.nsw.gov.au - Contact the Secretary’s ACEC to ask if they would be prepared to supervise the type of project you plan to undertake. Please complete the Secretary’s ACEC Access Application Form and submit it to secretary.acec@dpird.nsw.gov.au. Please note, this is not a project application form, it is a form requesting to use the Secretary’s ACEC. Please wait for a response to this application prior to submitting and paying for a project application.
- Submit the completed relevant Project Application Form via secretary.acec@dpird.nsw.gov.au
- If you have not used the Secretary’s ACEC as a Principal Investigator (PI) previously you will need to provide:
- a current CV
- two written references from people you have worked with regarding your experience in the type of research/surveys you plan to undertake. Links to some reference templates are provided below. Please note the use of the reference template is not mandatory.
- a certificate as proof of training in animal research ethics within the last 3 years. If you have not undertaken training in this time, we recommend the ANZCCART Compass Phase 1 training (all modules).
- A signed Secretary’s ACEC Sharing Agreement
- If you have previously been a PI on a project overseen by the Secretary’s ACEC and are submitting a new or renewal application, you must provide a certificate as proof of training in animal research ethics within the last 3 years. If you have not undertaken training in this time, we recommend the ANZCCART Compass Phase 1 training (all modules).
- Pay the prescribed $500 fee, as explained in the application form, via the online portal. Please wait to be advised when you need to pay. https://forms.bfs.dpi.nsw.gov.au/forms/22913
- The ACEC will then consider your application at the next scheduled meeting, and if approved, an Animal Research Authority (ARA) will be issued for the next 12 months. You can then commence the project after you have received the ARA.
Forms you will need to submit over the life cycle of each project
Please see the Secretary’s ACEC Forms section below to access the specific forms you need.
New project applications
- You must submit the relevant New Project Application Form, including online payment
- If it is your first time applying for a project under the Secretary’s ACEC, you will also need to submit:
- A CV
- two written references regarding your previous animal research experience and supporting your application. Please note the use of the reference templates below is not mandatory.
- A signed Secretary's ACEC Sharing agreement.
- A certificate as proof of training in animal research ethics within the last 3 years. If you have not undertaken training in this time, we recommend the ANZCCART Compass Phase 1 training (all modules).
- The relevant Annual Report Form, including online payment.
- The relevant Annual/Final Report Form
- The relevant New Project Application Form, including online payment.
- a certificate as proof of training in animal research ethics within the last 3 years. If you have not undertaken training in this time, we recommend the ANZCCART Compass Phase 1 training (all modules).
Annual renewal of Animal Research Authority
Animal Research Authorities only last for 12 months. In order to legally continue your project, each year throughout the project approval period, you must submit an Annual Report for ACEC review at a scheduled ACEC meeting prior to the Animal Research Authority expiry date. This will often mean your annual report will need to be submitted before the full 12 month period has elapsed.
Please note, projects will be closed if annual reports are more than 3 months late, on the assumption that project approval is no longer required. New full application submission and fee payment will be required to restart a closed project.
Project renewal at the end of the project 3 year approval period
If you wish to continue with an approved project after the project approval period expires, you must submit for AEC review at a scheduled ACEC meeting prior to the Animal Research Authority expiry date:
Please note, any project approvals, including modifications, do not roll over into the next project approval period. The new application is a complete restart and reset of the project
- A Final Report Form
Project completion
After you finish your project, you must submit to the ACEC for review:
Project cycle and submissions required
Payment required for application for an Animal Research Authority
Project where applications (new or renewal) were approved prior to 1 February 2025
An annual fee of $100 applies to each project. This payment was made when applying for a new project or renewal of an existing project at the end of the project approval period and when submitting an annual report for each project.
Please pay via the online portal when you are about to submit your annual report.
Project where applications (new or renewal) are approved after 1 February 2025
Please note this applies to all applications submitted after the submission cut off deadline (11 November 2024) for the 2 December 2024 ACEC meeting.
A fee of $500 applies to each project for the project approval period. This fee is paid at the time of applying for project approval. The usual project approval period is 3 years. if you wish to renew a project when the project approval period is due to expire, a new project application needs to be submitted, and a new $500 fee will apply. Annual Reports must be submitted for ACEC review and approval, however, no annual fee for the review of those reports applies.
Please note the $500 covers the application for ACEC review, and if approved, the subsequent review of Annual Reports and annual issuing of Animal Research Authorities, however, submission and review of a project application does not guarantee ACEC approval.
Please pay via the online portal when you are about to submit your new or renewal project application.
Fees for Authorities and Accreditations to allow research to be undertaken on animals under the Animal Research Act 1985 have been approved as exempt from GST. Division 81
A receipt for your payment will be emailed to you. Please attach the receipt pdf to the email when you are sending your application.
Annual Animal Use Reporting by Researchers
Under the NSW Animal Research legislation, details of animal use in research and teaching must be submitted to the NSW DPIRD annually by all holders of Animal Research Authorities. The information is collected because it is required by law and is used by NSW DPIRD for administration of the Act and is stored securely within NSW DPIRD.
Collation and submission of this animal use information for projects approved by the Secretary’s ACEC is done by the ACEC on behalf of the Accredited Animal Research Establishments and independent researchers. However, the Principal Investigator of a project must include required information in their Project Applications and Annual and Final Project Reports. Details of the annual animal use for each project is taken from the Annual or Final Report submitted for each project.
Please refer to Guidance for researchers using the Secretary’s ACEC regarding reporting animals used in research annually.
For more information on animal use reporting in NSW, please refer to Form L on the Animal Ethics Infolink. Please note that researchers using the Secretary's ACEC do not need to complete and submit Form L - this is done by the ACEC.
Does your project involve wildlife?
Vertebrate wildlife fauna research that includes capture and non-capture techniques that may impact on the animal’s behaviour, wellbeing or health is considered animal research and as such requires application for approval by an Animal Ethics Committee. This may include, but is not limited to activities such as:
- fauna surveys for environmental impact assessments
- conservation or threatened species assessments
- fauna monitoring programs
- ecological, biodiversity and distribution surveys
- pre-clearance surveys
- surveys prior to relocation
- radiotracking or tagging wildlife
- translocation when data is being collected after relocation
- wildlife capture, measurement, sampling and/or banding
- camera trapping using white flash and/or lures
- spotlighting and other non-capture observation methods
- behavioural studies using wildlife.
Spotter catcher/relocation work or the benefit of animal welfare around the time of land clearance is not considered animal research under the Animal Research Act 1985 and does not require Animal Ethics Committee approval. Surveys to determine presence of animals pre or post land clearance are animal research.
For projects involving wildlife, please visit National Parks and Wildlife to ensure the appropriate licenses and permits are obtained.
Guidance for submitting notifications when using subcontractors
Animal Research collaborations between Contractors and Subcontractors.
Processes described below assist in reducing duplication and provide streamlined approach for collaborative contracted research activities.
Scenarios | Sub scenarios | Options for ARAs | Who submits the Notification Form to the ACEC | Who includes the animal use in their Annual Report to the ACEC |
1. Both have approval for the specific procedure(s) under their own ARAs | 1.1 People from the Subcontractors ARA only are working on the specific project. | Subcontractor submits the notification with the Contractor copied into the email to the Secretary’s ACEC. | Subcontractor | |
1.2 People from both Contractor and Subcontractors ARAs are working on the same specific project doing same or different procedures. | Contractor submits the notification with the Subcontractor copied into the email to the Secretary’s ACEC. The Subcontractor(s) are added to the Notification Form under the Subcontractor section. | Contractor | ||
2. Both have ARAs but only the Contractor has approval for the specific procedure(s) under their own ARA | The Subcontractors are added as Associate Investigators to the Contractor’s ARA via a modification application for ACEC approval. | Contractor submits the notification with the Subcontractor copied into the email to the Secretary’s ACEC. The Subcontractor(s) are added to the Notification Form under the Associate Investigator section. | Contractor | |
The Subcontractor can add the procedures to their own ARA via a modification application for ACEC approval. | Contractor submits the notification with the Subcontractor copied into the email to the Secretary’s ACEC. The Subcontractor(s) are added to the Notification Form under the Subcontractor section. | Contractor | ||
3. Both have ARAs but only the Subcontractor has approval for specific procedure(s) under their own ARA | Contractor does not add the Procedures to their own ARA. | Subcontractor submits the notification with the Contractor copied into the email to the Secretary’s ACEC. | Subcontractor | |
The Contractor adds the procedures to their own ARA via a modification application for ACEC approval. | Contractor submits the notification with the Subcontractor copied into the email to the Secretary’s ACEC. The Subcontractor(s) are added to the Notification Form under the Subcontractor section. | Contractor | ||
4. Only the Contractor has an ARA, including the procedure(s) | The Subcontractors are added as Associate Investigators to the Contractor’s ARA via a modification application for ACEC approval. | Contractor submits the notification with the Subcontractor copied into the email to the Secretary’s ACEC. The Subcontractor(s) are added to the Notification Form under the Associate Investigator section. | Contractor | |
The Subcontractor applies for their own ARA, including the procedure(s) | See Scenario 1 | See Scenario 1 | ||
5. Only Subcontractor has an ARA, including the procedure(s) | Contractor does not apply for an ARA. | Subcontractor | Subcontractor | |
The Contractor applies for their own ARA, including the procedure(s) | See Scenario 1 | See Scenario 1 |
Secretary's ACEC Forms
The current forms and templates for the Secretary's ACEC are available below. Any new forms will be uploaded here. Please check this page to ensure you have the latest version of the form prior to making your submission.
Secretary's ACEC Sharing Agreement
Under the Australian code for the care and use of animals for research purposes 8th Edition 2013 clauses 2.6.2 and 2.6.3, when an establishment or independent investigator seeks to access an external AEC, the use of the AEC must be based on a formal agreement.
If you wish to apply to use the Secretary’s ACEC for the first time to oversee an animal research project, please refer to the Guidance for new researchers applying to use the Secretary's Animal Care and Ethics Committee webpage.
If you are eligible to use the ACEC, please complete the agreement form below and submit it along with your new application form.
New and 3 year Project Renewal Application Forms
Which application form to use
Please use one of the forms below when applying for a new project.
The same forms are also used to apply to renew a project to continue for another 3 years at the end of its current 3 year approval period. Please remember if you are renewing a project at the end of its 3 year approval period to also submit a final report for the existing project approval period.
Fauna Surveys
Fauna Survey Project Application Form
Use this form when applying to do vertebrate wildlife fauna research that includes capture and non-capture techniques that may impact on the animal’s behaviour or health. This may include, but is not limited to activities such as:
- fauna surveys for environmental impact assessments
- conservation or threatened species assessments
- fauna monitoring programs
- ecological, biodiversity and distribution surveys
- pre-clearance surveys
- surveys prior to relocation
- radiotracking or tagging wildlife
- wildlife capture, measurement, sampling and/or banding
- camera trapping using white flash and/or lures
- spotlighting and other non-capture observation methods
- behavioural studies using wildlife.
Please note: Spotter catcher/relocation work is not considered animal research under the Animal Research Act 1985 and is does not require animal ethics committee approval. Surveys to determine presence of animals pre land clearance are animal research and requires animal ethics and ethics committee approval.
To assist in completing the Fauna Survey Application form, please refer to the Guide for completing the Secretary's ACEC Fauna Survey Application webpage
General Research
General Research Project Application Form
Use this form when conducting animal research using non-wildlife animals for recognised research purposes. This may include, but is not limited to:
- clinical trials
- therapeutic efficacy/safety trials
- food trials
- research testing biological parameters;
- animal behavioural research
- collection of biological products for commercial purposes.
If you are using privately owned animals, please submit an owner information and consent form template that you will be using, along with your application. You can develop your own template or use the template below, adding the relevant details for the project.
Owner information and consent form template - General Research
Educational Activities
Educational Activities Project Application Form
Use this form when applying to use animals for the purpose of education/training. This may include, but is not limited to:
- ultrasound or dental education workshops
- animal handling courses
- basically, delivery of training of people using animals when this is not part of normal animal husbandry or required for veterinary management of the animal.
If you are using privately owned animals, please submit an owner information and consent form template that you will be using, along with your application. You can develop your own template or use the template below, adding the relevant details for the project.
Owner information and consent form template - Educational activities
Animal Supply Supervision
Animal Supply Supervision Application Form
This form should be used by establishments wanting to apply for the Secretary’s ACEC to supervise their Animal Supply Licence in order to obtain, breed, keep and supply animals for use in connection with animal research.
For further information on the requirements and exemptions relating to animal supply please refer to the Animal Ethics Infolink.
Project Modification Application Form
Any proposed change to a project must be submitted to an ACEC for approval. This includes, but is not limited to changes to personnel, procedures, housing, transport and location, if a specific location has been approved in the project application. Please note that modifications must be approved by the ACEC prior to the changes being implemented.
Annual and Final Report Forms
Please remember that although projects are generally approved for a 3 year period, Animal Research Authorities are only issued for maximum of 12 months at a time.
In order to be compliant with the NSW Animal Research legislation, Annual Reports must be submitted to the ACEC in time for the ACEC to be able to review and approve it at a meeting prior to the project Animal Research Authority expiring. Following ACEC approval of the report a new Animal Research Authority will be issued for the following 12 months.
Projects that were approved prior to 1 January 2025
Please remember that when you submit an Annual Report, you also need to pay the annual fee of $100 via the online portal below. A receipt will be emailed to you.
Projects that were approved after 1 January 2025
No annual fee is required.
If you have a condition on your Animal Research Authority that you need to submit an Annual Register if you have had 'Other participants' or Subcontractors not listed on your current Animal Research Authority work on your project over the last 12 month reporting period, please complete and submit along with your Annual Report the relevant Annual Register of 'Other Participants' Form.
Forms
- General Research Project Annual and Final Report Form
- Fauna Survey Project Annual and Final Report Form
- Educational Activities Project Annual and Final Report Form
- Annual Register of Other Participants and Subcontractors Form - Fauna Survey Projects
- Annual Register of Other Participants and Subcontractors Form - General Research and Educational Activities Projects
Animal Supply 6 Monthly Reporting Forms
Establishments that use the Secretary’s ACEC to supervise their Animal Supply Licence in order to obtain, breed, keep and supply animals for use in connection with animal research must submit a report regarding activities under their ACEC approval on a 6 monthly basis. Reporting is required for the periods 1 January to 30 June and 1 July to 31 December each year.
Please use one of the below forms below for reporting to the ACEC.
Project Notification Forms
If your approved project has a condition that requires you to notify the ACEC of specific details prior to commencement of a survey, training course or research collaboration etc, please use one of the relevant forms below and submit to secretary.acec@dpird.nsw.gov.au
Unexpected Adverse Event and Non-Compliance Report Forms
Any unexpected adverse events that occur in an approved project must be reported to the ACEC as promptly as practicable.
Unexpected Adverse Events
What is an Unexpected Adverse Event?
The Code defines an Unexpected Adverse Event as: an event that may have a negative impact on the wellbeing of animals and was not foreshadowed in the approved project application.
The Secretary’s ACEC understands that adverse events may occur despite every attempt to mitigate their occurrence. They believe unintentional and unexpected are the same thing. The ACEC will use a ‘common sense approach’ when reviewing adverse events. When writing project applications, the committee encourage researchers to adopt a risk assessment approach in order to best mitigate for adverse events.
An unexpected adverse event may result from different causes, including but not limited to:
- death of an animal, or group of animals, that was not expected
- adverse effects following a procedure or treatment that was not expected
- adverse effects in a larger number of animals than predicted during the planning of the project or activity, based on the number of animals actually used, not the number approved for the study
- a greater level of pain or distress than was predicated in the planning of the project or activity and
- power failures, inclement weather, emergency situation or other factors external to the project or activity that have a negative impact on the welfare of the animals.
Reporting timeframe for an adverse event
Reporting of an adverse event should be carried out as soon as practicable to the ACEC Executive Officer, ideally within 72 hours of the event.
When to do a Post Mortem Examination?
- When no cause of death is readily apparent.
- When no cause is apparent and the health of more animals is under threat.
- When there is any possible public health implication.
Guidance for reporting Unexpected Adverse Events
For more guidance regarding unexpected adverse events.
Please submit a completed Unexpected Adverse Event Report Form to the ACEC as soon as practicable after the event and preferably within 72 Hours of the event.
Non-Compliance Events
What is a non compliance?
The Secretary’s ACEC recognises that non-compliance events may be unintentional, due to misunderstanding or miscommunication between various parties of what the ACEC has approved. There are also instances where non-compliance may be intentional. The ACEC would like to help educate researchers to improve their compliance with the legislation and thereby improve the wellbeing of animals used in research and teaching.
Examples of non-compliance may include:
- using trapping methods that were not included in the project application and therefore not approved by the ACEC
- using species of animals not approved by the ACEC
- using higher numbers of animals than were approved by the ACEC
- using procedures that were not approved by the ACEC
- conducting an educational activity using live animals that was not approved by the ACEC.
When to submit a Non-Compliance Report Form?
An animal research non-compliance event should be reported if you become aware of non-compliance in an animal research project overseen by the Secretary's ACEC. It is in the researcher’s or businesses' best interest to self-report any non-compliance as it helps work out how it occurred, how to regain compliance and how to prevent it occurring again. It also allows the ACEC to investigate, if required, and provide advice for future prevention and how to be compliant with the NSW Animal Research legislation again.
Please submit a completed Non-Compliance Self Reporting Form to the ACEC as soon as practicable after the event and preferably within 72 Hours of the event.
Guidelines, Procedures and Information Sheets
- Secretary’s ACEC Trapping Policy
- Guidance for researchers on reporting animals used in research
- NSW Animal Research Act 1985
- NSW Animal Research Regulation 2021
- Australian code for the care and use of animals for scientific purposes 8th Edition 2013
- Biodiversity Conservation Act 2016
- National Parks and Wildlife Act 1974
- Threatened species Conservation Act 1995
- NSW Scientific Licences
- Animal Research Review Panel’s guideline ‘Wildlife surveys’
- Animal Research Review Panel’s ‘Use of Pitfall traps’ guideline
- Animal Research Review Panel’s Emergency Procedures
- Animal Research Review Panel’s radio tracking and GPS tracking guideline
- NSW Environment, Energy and Science Guidelines for carrying out a survey
- Best Practice Guideline for the use of Koalas in Scientific Research
- Hygiene protocols for the control of diseases in Australian frogs NPWS 2008
- Hygiene protocols for the control of diseases in Australian frogs James Cook University 2011
- Threatened species survey & assessment guidelines: field survey methods for fauna (Amphibian)
- Threatened biodiversity survey and assessment – Guidelines for developments and activities (2004 working draft)
- Australian Government Survey guidelines for Australia’s threatened mammals
- A guide to the care and use of Australian native mammals in research and teaching (NHMRC, 2014).
- NSW Drones in national parks policy
- Protecting trapped animals from heat exposure: the influence of shading on temperature with small metal box traps
- Supplement to above article
- Australian Bird Banders Manual (Lowe 1989)
- Euthanasia guidelines AVMA 2020
- ANZCCART Euthanasia of Animals Used for Scientific Purposes (under revision)
Secretary’s ACEC Circulars
The ACEC issues Circulars to the researchers and accredited establishments whose projects they oversee to increase awareness of obligations under the NSW Animal Research Legislation and the Australian code for the care and use of animals for research purposes, thereby helping ensure the wellbeing of animals used for research.
Secretary's ACEC meetings and submission deadlines
Please find below the meeting and submission deadline dates for 2023. All submissions must be made by the submission deadline to be considered at the relevant meeting. Submission to the Secretary’s ACEC can be sent to secretary.acec@dpi.nsw.gov.au
For any further information, please email secretary.acec@dpi.nsw.gov.au
Meeting Dates 2025
Meeting dates | Submission Deadline |
|---|---|
3 February | 17 January |
24 March | 3 March |
5 May | 14 April |
| 16 June | 26 May |
28 July | 7 July |
20 October | 29 September |
14 October | 23 September |
1 December | 10 November |

