Soil acidity and liming 4th edition
Disclaimer: The information contained in this publication is based on knowledge and understanding at the time of writing (Dec 2020). However, because of advances in knowledge, users are reminded of the need to ensure that information upon which they rely is up to date and to check currency of the information with the appropriate officer of the Department of Primary Industries or the user’s independent adviser.
This publication replaces Soil acidity and liming. Agfact AC.19, 3rd edition 2005 by Brett Upjohn, Greg Fenton & Mark Conyers. Some technical content is adapted from this earlier edition
Soil Acidity in NSW
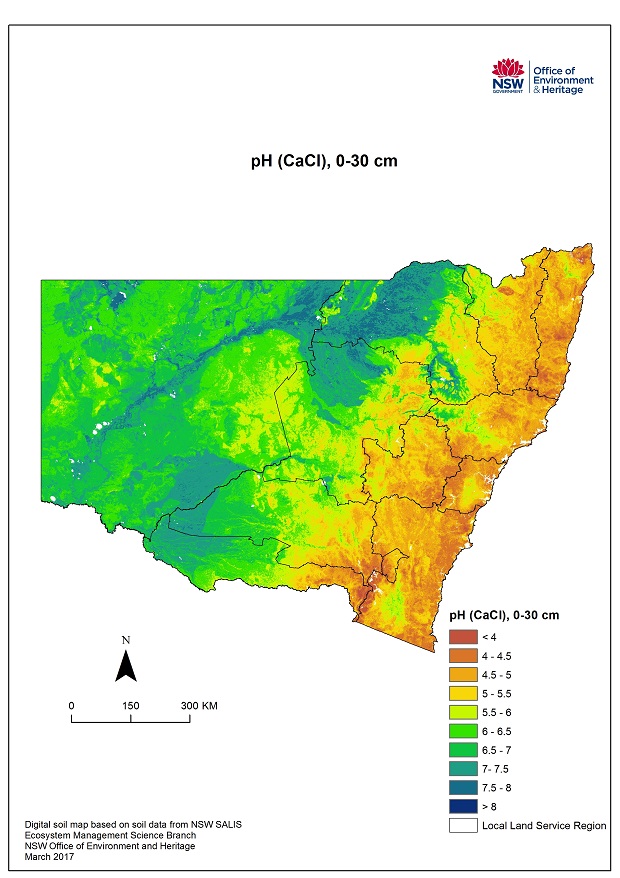
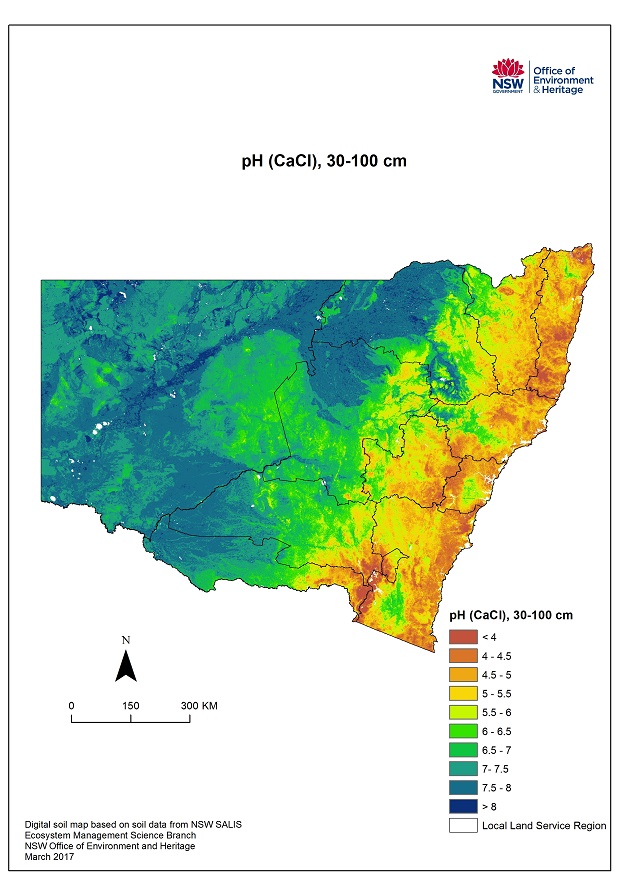
Maps courtesy of Environment, Energy and Science | Department of Planning, Industry and Environment
OEH 2018. Digital soil mapping of key soil properties over NSW, version 1.2. Technical Report, NSW Office of Environment and Heritage, Sydney. (prepared by J. Gray)
Acidic soils are an impediment to agricultural production. More than half of the intensively used agricultural land in NSW is affected by soil acidity. While soil acidification is a natural consequence of weathering processes of soil formation, today's agricultural practices increase acidification thus placing limitations on future production. In some areas this results in permanent degradation of NSW soils.
Acidic soils are an impediment to agricultural production. More than half of the intensively used agricultural land in NSW is affected by soil acidity. While soil acidification is a natural consequence of weathering processes of soil formation, today's agricultural practices increase acidification thus placing limitations on future production. In some areas this results in permanent degradation of NSW soils.
It has been estimated that the cost of soil acidity in terms of annual lost agricultural production in Australia are A$1,585 million 1. And these costs are most pronounced in the high‐rainfall regions of New South Wales, Victoria and Western Australia where yield gaps have been estimated to be 0.1–0.2 t/ha/yr 2.
The National Land & Water Resources Audit (2001) identified soil acidity as the most serious land degradation issue for Australian agriculture and estimated that 50 million hectares of Australia’s agricultural land are already experiencing impacts from soil acidity in surface layers and a further 23 million hectares in subsurface layers 3. Subsequent State of the Environment reporting shows soil acidification continues to have an increasing impact on soil condition 4.
The maps here give a broad overview of pH levels at two soil profile depths for NSW. However in order to diagnose and manage an acid soil a soil test from a reputable laboratory is required of the individual paddock or landscape element(s).
What is an acidic soil?
- Technically an acidic soil is one with a soil pH (CaCl2) below 4.8 in the root zone (0-20cm).
- A soil pH (CaCl2) between 5.5 and 8.0 provides the best conditions for most agricultural plants.
- Sometimes soil acidity is a natural characteristic of the soil which may be difficult to ameliorate economically.
How can I tell if soil acidity is a problem?
Although reduced production is often the end result, the visual signs of soil acidity are more subtle than the clearly visible symptoms of other soil degradation. A soil test is the most reliable way to assess if a soil is acidic.
The only reliable way to determine if a paddock has an acidic subsurface soil is to sample and analyse the 0-5cm, 5-10cm, 10–15 cm, 15-20 cm, and 20–30 cm layers.
A comprehensive chemical analysis of the surface soil layers will give information to assist in determining if a crop or pasture will be affected by acidity. In order to assess whether acid sensitive crops will be affected by subsurface acidity, it is recommended that soil be tested for pH below 10cm, especially where reduced tillage has been used .
Read Signs and symptoms for other indicators
Testing
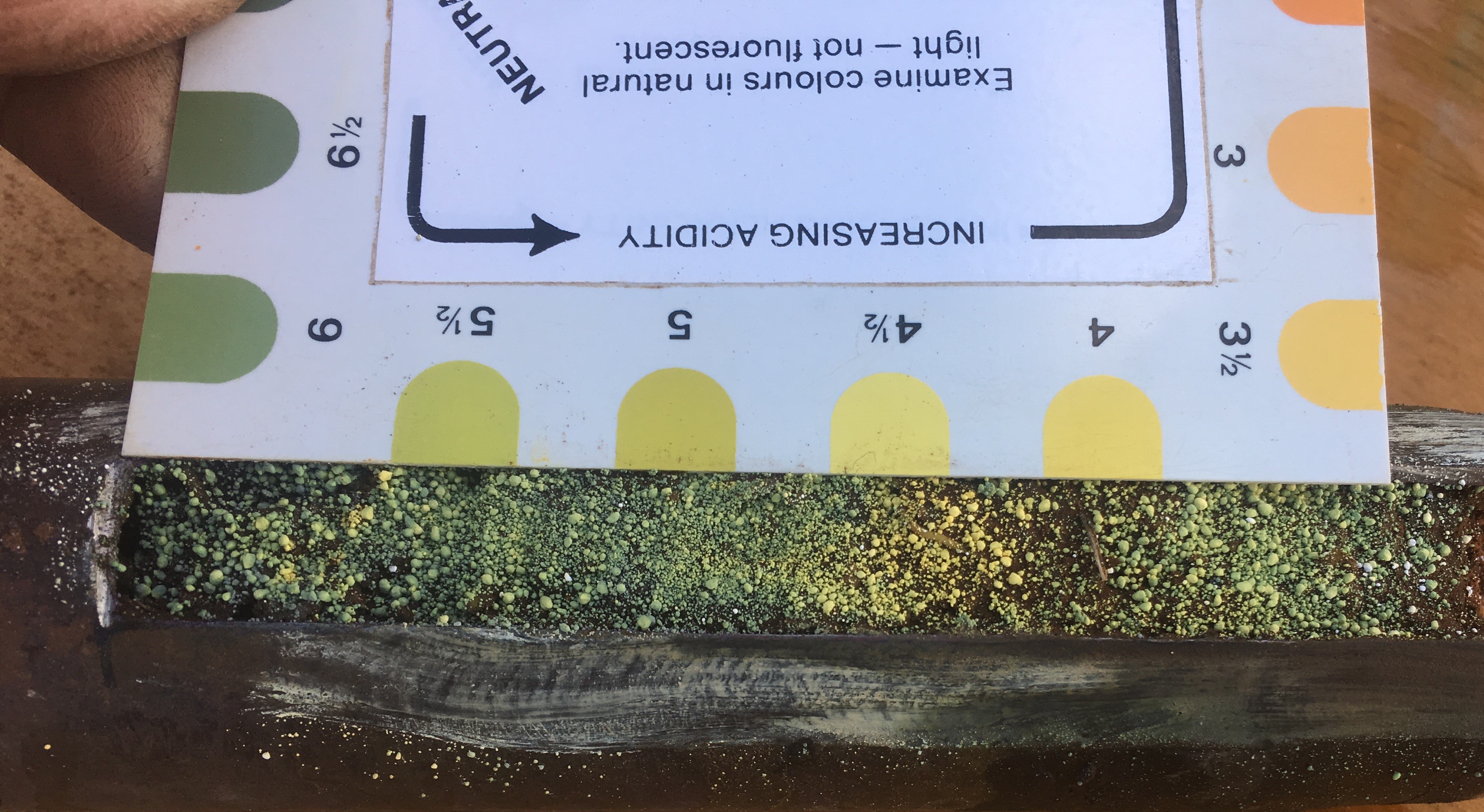
A field test using a field soil pH kit or meter is a good place to start. It is simple to carry out and will indicate if further investigation with a full laboratory test is required and at what intervals samples should be taken. This level of detail is required to decide which management strategies to employ and helps in species selection.
There are two accepted ways to measure soil pH: one in water and one in calcium chloride. The pH measured in calcium chloride is on average 0.5 to 0.8 units less than pH measured in water, although the difference can vary from nil to 2.0 for different soils, soil textures and salt content. In this publication pH is nominated as pH(CaCl2)
REMEMBER:
The pH scale is logarithmic, which means that every point is an order of magnitude different from that below or above it. For the pH scale in particular this means that a pH of 4 is 10 times more acid than 5 and 100 times more than a pH of 6.
Field colourmetric test uses the pH in water method
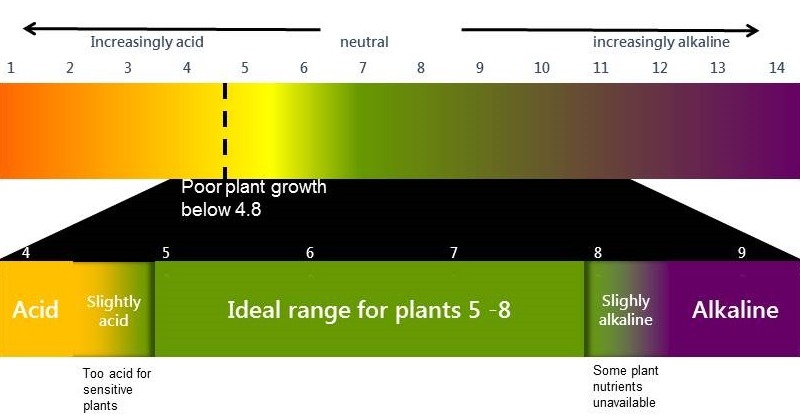
Plant growth and the pH (CaCl2) scale
If the pH(CaCl2) drops below 5.0, plants that are highly sensitive to acidity, such as some legumes and barley, are adversely affected. Plants that are more tolerant of acidity continue to grow normally until the pHCaCl2 falls below 4.8. However it is important to note that often Rhizobium bacteria are more sensitive to soil acidity than the host plant. This means nodulation and nitrogen fixation will be impaired before the host plant is affected (see). Not only does this have a negative effect on the legume itself but the lack of effective nodulation reduces the nitrogen contribution of legumes in both crop and pasture systems.
Below pHCaCl2 4.4 most plants, except the very highly acid tolerant plants like oats, narrow leaf lupins and the native pasture grass Microlaena spp, show a significant reduction in production. Even in tolerant species productive capacity will be affected by soil acidity. Even if soil acidity does not result in plant death, production will be suboptimal.
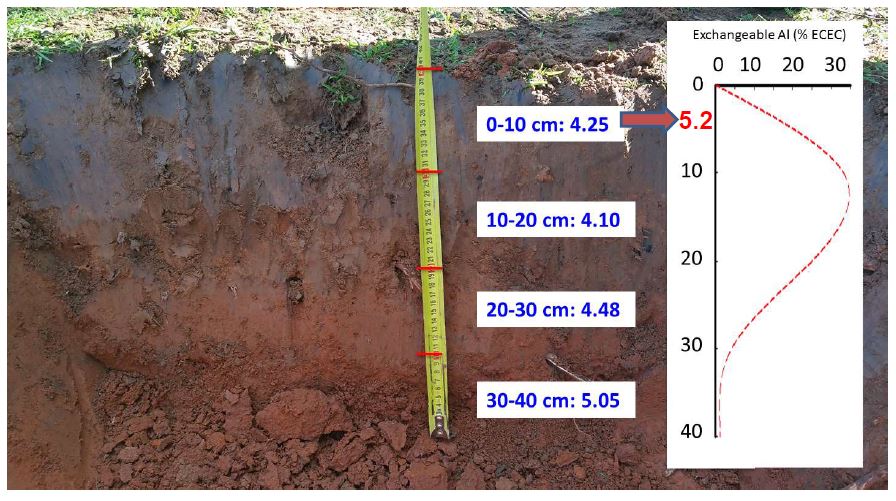
Soil acidity throughout the profile
Research has shown the process of soil acidification within the soil profile results in a stratification of soil pH. This means that the topmost surface of the soil profile (0-5cm) will often have a higher pH than those layers below. But it is these deeper layers that are important for plants to achieve their productive potential, as it is where most plant roots grow. When acidification is not managed it becomes more and more difficult to remediate. The greater the depth of the acidic layer, the greater the effect on plant growth and the more difficult it is to correct. Untreated subsurface soil acidity results long term degradation of the soil.
Whilst models showing the chemical process of soil acidification are very good at explaining the chemical causes, these models do not account for the three dimensional nature of soil in the paddock. They do not account for movement of acidity within the soil profile nor the fact that a soil profile is not uniform. This spatial variation results in stratification of soil pH down the soil profile in increments as small as 0-5cm, 5-10cm, 10-15cm etc which can be exacerbated by modern farming techniques such as reduced tillage. For further information on causes of soil acidity and stratification see Causes and video below.
Signs and symptoms of an acid soil
Low pH in soil is a problem for agriculture and the environment because it can affect plant growth through its effect on plant nutrients and soil biology
Change in nutrient solubility and nutrient availability to plants
Some nutrients may reach toxic levels, while others can become insoluble (therefore unavailable), leading to deficiencies. The changes in the solubility of plant nutrients associated with increasing soil acidity are:
- Increased aluminium (Al3+) in the soil solution causing stunted root development in crops and pastures. Stunted roots result in reduced capability to access soil moisture and reduced nutrient uptake. In legumes resulting in reduced nodulation and nitrogen fixation, which may also reduce survival.
- Increased manganese (Mn2+) in the soil solution, causing reduced growth in some plants in some soils (with toxicity symptoms in susceptible plants).
- Reduced solubility of molybdenum, phosphorus, and access to calcium, while increased aluminium in the soil solution facilitates the movement of calcium and magnesium down the soil profile where it is less accessible.
Some plants are more sensitive to aluminium than to manganese and vice versa. For example, white clover is tolerant of aluminium but sensitive to manganese. Often the cumulative effects of low pH and various toxicities/deficiencies result in the greatest damage.
The degree to which soil acidity affects nutrient availability and therefore plant health and growth depends on pH level, soil type and soil organic matter levels.
Changes in biological activity
Sometimes the effect of acidic soils on the growth and production of crops and pastures is not direct, but rather through the effect on soil micro-organisms, that in turn affect plant growth. Soil organisms are influenced by soil pH.
Soil pH influences both survival and functioning of Rhizobia.
The fungus that causes the root disease Take-all in cereals, Gaeumannomyces graminis var.tritici, is most active in soils with a pH(CaCl2) greater than 4.8, and has a low level of activity in soils with a pH less than 4.6. Liming greatly increases the activity of Take-all.
Farmers and land managers will see evidence of the above expressed as:
- reduced yields from acid sensitive crops and pastures
- poor establishment of pastures
- failure of perennial pastures to persist
- poor legume productivity and nodulation
- increased need for fertiliser N in crops following legume-based pastures due to poor nitrogen fixation by pasture legumes
- poor regeneration of annual legume species
- reduced tolerance of crops and pastures to environmental stresses such as waterlogging, drought, disease and herbicide damage
- increased incidence of acid tolerant weeds) e.g. sorrel
Remember general signs that may point to soil acidity can also be the result of other land degradation issues:
* Reduced yields from acid sensitive crops and pastures
* Poor establishment of perennial pastures
* Failure of perennial pastures to persist
What causes soil acidification?
- Acidification of the soil is a slow natural process and part of normal weathering.
- Many farming activities cause an increase in the rate of acidification of the soil.
- Changes in soil pH under agricultural use are measured in tens of years rather than thousands of years as in the natural environment.
There are four ways that agriculture contributes to the accelerated acidification of the soil and these are:.
- Removal of produce
- Use of fertilisers containing ammonium or urea
- Movement of nitrate nitrogen sourced from nitrogen fixation or from ammonium fertilisers
- Build-up of soil organic matter.
 In some older texts the removal of “base” cations, that is calcium, magnesium, potassium and sodium, is given as a cause of soil acidity. This is misleading, and in a similar vein acidity cannot be corrected by applying calcium.
In some older texts the removal of “base” cations, that is calcium, magnesium, potassium and sodium, is given as a cause of soil acidity. This is misleading, and in a similar vein acidity cannot be corrected by applying calcium.
Rate of acidification
It is possible to estimate the rate of acidification for a given paddock enabling budgeting for future liming programs, but it is best to use lab test results of your soil to determine liming requirements.
Rainfall is the climatic feature that has the greatest effect on the rate of soil acidification as it influences:
* plant productivity (which includes the amount of nitrogen fixed by legumes)
* movement of nitrate nitrogen
These factors interact in their influence on the rate of acidification.
In the past there has been a misconception that perennial pastures can be used to prevent soil acidification. This is not the case for reasons previously explained in the section What is an acidic soil? and Removal of produce above.
How do I manage acidic soils?
Prevention and amelioration
Management needs to aim at maintaining a profitable farming system and improving soil condition to minimise the degradation caused by soil acidification.
Managing acid soils is most important for the long term sustainability of farming systems and the soil resource.
Effective management of acid soils will involve a combination of these three management approaches:
- Minimise acidification
- Apply a liming product (amelioration)
- Use acid tolerant crop and pasture varieties
It is understood that agricultural production is an acidifying process. For your chosen production system, management options available to minimise soil acidification include the types, rate, placement and timing of fertiliser application(s).
The application of limestone or other alkali material that neutralises soil acidity is the only practical amelioration technique. Its effectiveness is determined by product quality, rate and application method.
Acid tolerant plant species can be used to maintain some production whilst implementing amelioration. It is important to remember that acid-tolerant plants can also be responsive to increases in soil pH. In the case of legumes it is important to consider not only plant impacts but also the possible effects of soil pH on nitrogen fixation.
There can be considerable hidden costs in choosing a tolerance rather than amelioration pathway to manage soil acidity.
Minimise acidification
Where soils are at risk of becoming acidic the future impact of soil acidity can be reduced, but not eliminated, by slowing the rate of acidification. This can be achieved by choosing fertilizers that are less acidifying and managing the movement of nitrate nitrogen in the soil.
1. Use less acidifying fertilisers
2. Reduce the movement of nitrate nitrogen
Apply Lime
To get the best effect, high quality lime should be finely crushed, evenly spread and incorporated into the part of the soil where acidity is a problem (or as deep as practicable).
Lime moves very slowly through the soil. Incorporation is recommended where possible and where erosion risk is low. Where soil acidity occurs at depth incorporation will hasten the liming effect.
Any liming needs to be done at least a season prior to sowing a sensitive crop to ensure lime has had time to react. Lime needs to be applied to the entire layer where the pH is suboptimal to ensure amelioration.
The amount of lime required should be determined using pH in CaCl2 soil test results from an accredited lab and the table below. Soil samples should be collected using a valid sampling strategy.
What is Lime?
The name used to describe any of several liming materials, including agricultural limestone and dolomite. In the building industry “lime” refers to calcium hydroxide (slaked lime)
Achieving a target pH
Historically lime was applied to achieve a soil pH of 5.2 (0-10cm) to remove the effect of toxic aluminium on plant production. However this will not stop acidification further down the soil profile, nor is it optimal for most commercial rhizobia strains. There are significant benefits when pH is maintained above 5.5.
Where acidity occurs below the depth of lime incorporation the neutralising effect will only progress deeper into the soil profile if the surface soil is maintained above pH(CaCl2) of 5.5
Checklist before applying lime to achieve a pH(CaCl2) of 5.5
To ensure that a response to liming is likely, particularly when putting in a lime trial or test strip, make sure:
- Areas and depths of acid soil (<5.5) using test results (eg at 0-5, 5-10, 10-15, 15-20 cm etc depth intervals) are identified.
- Soil pH is the most limiting manageable soil factor to plant growth.
- The liming product to be used is sufficiently fine and with a high neutralising value, or is the most cost effective product available.
- The lime rate is sufficient to achieve a soil pH(CaCl2) of 5.5
- Take-all has been controlled when planting wheat or triticale.
- The timeframe is long enough for applied lime to raise the pH to the desired level, especially where growing plants sensitive to acidic soils.
Grow acid tolerant crops and pastures
If a paddock is already acidic, particularly where both the surface and subsurface soils are acidic, the economic and offsite impacts can be reduced by growing acid tolerant crops and pastures.
However acidification will continue despite the use of acid tolerant plants unless other management options are used to ameliorate soil acidity and or prevent further acidification.